U n i v e r s i t é Y O R K U n i v e r s i t y
ATKINSON FACULTY OF LIBERAL AND PROFESSIONAL STUDIES
SCHOOL OF ANALYTIC STUDIES & INFORMATION TECHNOLOGY
S C I E N C E A N D T E C H N O L O G Y S T U D I E S
NATS 1800 6.0 SCIENCE AND EVERYDAY PHENOMENA
ATKINSON FACULTY OF LIBERAL AND PROFESSIONAL STUDIES
SCHOOL OF ANALYTIC STUDIES & INFORMATION TECHNOLOGY
S C I E N C E A N D T E C H N O L O G Y S T U D I E S
NATS 1800 6.0 SCIENCE AND EVERYDAY PHENOMENA
Lecture 19: Science in the Kitchen
| Prev | Next | Search | Syllabus | Selected References | Home |
… at its most basic level, science is nothing more than
a way of answering questions about the things that happen to us every day.
It is not something separate from the natural world;
it is a way of looking at the natural world and trying to understanding it.
Russ Parsons, How to Read a French Fry (Houghton Mifflin Company, NY 2001).
Topics
-
To get things started, read On Food and Cooking – The Science and Lore of the Kitchen, completely revised and updated .
The author of the book reviewed here, Harold McGee, is probably the first cookbook writer who brought together rigorous science
and cooking experience. You may also want to listen to a two-part interview with McGee on PBS.
And here is a review which appeared in the Journal of Chemical Education.
… or consider a kitchen question that may require a little science to be answered: "Why can you stick your hand in
a 450-degree oven but not in 212-degree boiling water?" [ from How to Read a French Fry, p. 3 ]
Can you answer it? Is the 'little science' needed helpful, in the sense that it adds to, expands, generalizes
the kitchen wisdom we all have, more or less?

Giuseppe Arcimboldi (ca 1527 - 1593): Vertumnus
"Have you ever noticed that a whole onion smells different from one that's been cut? Have you ever wondered why? Here's the answer: Physically, an onion is 90 percent water, trapped in a fairly flimsy network of cellulose. Within that network is a subnetwork of smaller cells, called vacuoles. These vacuoles separate a variety of chemical components suspended in the water. It's only when the vacuoles are ruptured, either by cutting or by smashing, that these chemical components combine and then recombine again and again in a cascade of chemical reactions, creating the smell and taste we associate with raw onions. Most simply put, what happens is that the contents of these separate vacuoles combine to form a variety of sulfur-rich compounds called sulfonic acids. These acids in turn combine to form still more compounds that provide most of the fresh-cut onion character. What's more, this chain of reactions happens in a flash. It's a little miracle. In fact, not until the 1970s had science advanced to the point that it could begin to decipher what happens in that fleeting instant between the time your knife touches the onion and the fumes reach your nose."
Thus begins Russ Parsons' How to Read a French Fry (Houghton Mifflin Company, NY 2001). Now, while knowing the processes responsible for onion fumes may satisfy curiosity more than need, there are plenty of other cases where knowledge of the underlying biological, chemical and physical processes can be of great practical utility in the kitchen. Before examining some of these processes, let me mention that "Japanese researchers have discovered an enzyme that the onion uses specifically to create the tear-jerking chemicals.""Until this discovery, if scientists had used genetic modification to yield a tearfree onion, they probably would also have compromised the flavor, says Imai. Propanthial S-Oxide—the onion irritant behind all the culinary sobbing—was considered a by-product of the reactions that produce the onion's characteristic flavor compounds, Imai says. Instead, Imai's group found a new enzyme that works specifically to produce the irritant. This enzyme doesn't contribute to the reactions that lead to the onion-flavor compounds. Therefore, genetic engineering might modify an onion so that it lacks only this particular enzyme—and so remains full-flavored."
[ from Less Crying in the Kitchen: Tasty, Tearfree Onions on the Horizon ] -
Consider common eggs, for example, which, as everybody knows, are used in a great number of recipes.
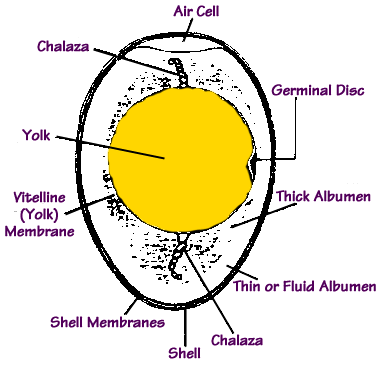
The Structure of the Egg
Eggs consist of three main parts: the shell, the white and the yolk. The shell is composed largely of calcium carbonate (calcite), and while impermeable to water, it is porous to air (and thus it can absorb odors). The white consists largely of a water suspension of proteins, and the yolk of a water mixture of proteins and fats. See, for example, Incubation and Embryology, which summarizes the overall composition as follows:water protein fat ash whole egg 74 13 11 1 white 88 11 0 -- yolk 48 17 33 1Let's see now how such basic data allows us to answer some simple cooking questions. Let's examine first what happens when you try to hard-boil an egg. As you know, one of the perennial problems is how long does it take to get the perfect hard-boiled egg? And here's a related one: how can you prevent the shell from cracking? Here are the answers:"Cover the egg with cold water and bring it to a boil. Immediately turn off the heat and leave the egg until the water has cooled enough to retrieve it. What you're doing is using a temperature curve. Picture a graph of the temperature of the water. It will be a bell curve—first it heats up, then it cools down. Bu cooking the egg this way, you're minimizing the time it spends at the extreme temperature that produces the iron/sulfur reaction while maximizing the amount of time it spends at the temperature that will firm the protein in the white and yolk (above 160°F [ or about 70°C ] )." [ from How to Read a French Fry, p. 123 - 124 ]
The iron/sulfur reaction mentioned above is the "chemical reaction between the traces of iron in the yolk and the traces of sulfur in the white," and is responsible for the gray-green color of an overcooked hard-boiled egg. This method also prevents the shell from cracking:"All egs contain an air pocket. When the air is heated quickly, as happens when you thoughtlessly dump an egg into boiling water, the air expands rapidly because of the heat and fractures the shell. But remember that an eggshell is a miraculous thing. It's hard yet porous. Heat the egg slowly and the air pocket will slowly leak out through the shell, relieving the pressure without cracking."
Let's move now to a more ambitious cooking project: making mayonnaise by hand. Mayonnaise is an emuslsion, i.e. "a stable and homogenous mixture of two liquids which do not normally mix (they are immiscible between themselves)." [ from Wikipedia ] Examples of emulsions are vinaigrette (oil and vinegar), milk, and butter (fats and water). Emulsions, if left alone, will soon separate into their ingredients. Their stability can be increased by adding emulsifiers. These are substances which either encase the droplets of one of the liquids in the emulsion, or bind to one or both of them, preventing them from merging together. One of the most common and simplest emulsifiers is prepared mustard:
[ from How to Read a French Fry, p. 124 - 125 ]"Add a tablespoon of mustard to that vinaigrette, and you'll notice a couple of things. First, the dressing will come out creamier and smoother than a simple vinaigrette. Second, it will last longer. Rather than having those droplets of oil simply dispersed in the water and gradually coming back together, the mustard surrounds the oil droplets with a very thin film. The dispersed oil drops may collide, but their mustard jackets keep them from hooking back up so readily. Rather than emulsifiers bringing different things together, which would seem to be the obvious thing they do, they are actually separators, keeping similar things apart."
Returning now to mayonnaise, as Russ Parsons points out, "probably the most useful emulsifier culinarily is the egg yolk." Yolk is a special emulsifier because it includes fatty acids whose molecules have the important property that one end is hydrophobic (repelled by water) and the other is hydrophilic (attracted by water). To prepare mayonnaise you need egg yolks, vinegar or lemon juice, and oil—plus much patience and some science.
[ from How to Read a French Fry, p. 126 - 127 ]"So when you beat together oil, water and one of these emulsifiers [ such as egg yolk ] something very interesting happens: you get an emulsion that will be very difficult to separate. Not only does the emulsifier coat the droplets, it locks them into a kind of network. In the case of egg yolks, the primary emulsifier is lecithin, which is one of the fats in the yolk."
Science also helps to explain some of the more mechanical aspects of the mayonnaise ritual. For example, we know that it is essential to proceed only when all the ingredients are at room temperature. The reason is that at room temperature the surface tension of the yolk and of the oil is reduced, and mixing is facilitated. You can refer back to our lecture on Foams and Bubbles.
[ from How to Read a French Fry, p. 127 ]"The ideal proportion of oil to yolk is between 65 and 75 percent [ … ] Use less oil than that, and you'l have a very stable emulsion, but it will flow more like a liquid than a sauce. Use too much more than that, and you will have a very thick sauce, but it will be highly unstable [ … ] There's too little emulsifier to hold that amount of oil [ … ] You need only enough to form a thin layer around each droplet of oil [ … ] Make sure you are working with the right size bowl too. If you try to make mayonnaise in too big a bowl, the egg yolk will be spread out too thin to begin the emulsion." [ from How to Read a French Fry, p. 128 ]
… and when you have mastered mayonnaise, you may want to read what Russ Parsons has to say about hollandaise. -
What is more satisfying and inspiring than a hot cup of coffee sipped quietly as you putter in your kitchen, or
as you look for inspiration in your trusted cookbook? So let's look right into one of those cups of coffee. Jay
Ingram promises we'll find "all kinds of interesting science." [ see Jay Ingram, The Science of Everyday Life, Penguin Canada, 1989, 1990, 1994 ]

A Coffee Plant
Here is Ingram's setup:"Fill a cup to the brim with very hot, black coffee and position it so there is light shining on the surface. A bright light bulb is fine, but early morning sunshine is even better. You'll see a pattern on the surface of the coffee like flagstones in a path: irregular whitish patches less than an inch across, bordered by narrow dark lines. They'll shift back and forth, changing their outline from moment to moment, but they don't disappear. These whitish areas bordered by dark lines are called convection cells, places where warm coffee is rising up from the bottom of the cup, and cooler coffee from the surface is sinking back down [ … ] The whitish areas are, amazingly, droplets of water suspended in the air. When hot coffee reaches the surface, individual molecules of water from the coffee evaporate, tearing themselves away from the surface. [ … ] Once airborne, these fast-moving water molecules quickly cool, slow down and coalesce to form a layer of tiny but visible droplets of water, just above the surface of the coffee. In any other circumstances, droplets would be pulled back down into the coffee by gravity, but in this case gravity is balanced by the buoyant force of the evaporating water molecules, so these droplets hang there, suspended less than a millimeter above the surface of the coffee."
[ from Jay Ingram, The Science of Everyday Life, p. 25 - 27 ]
Convection Cells in Coffee
If the cup had perfectly smooth bottom and walls, and the coffee in the cup were only about 1 mm deep, and uniform, steady heating were applied to the bottom of the cup, then you might be able to see a much more orderly convection pattern, such as the one illustrated below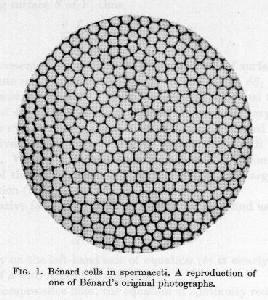
Bénard Convection Cells
These patterns (cells) are called Rayleigh-Bénard cells, in honor of the two physicists who studied them first. So far we have used only black coffee. What happens if you like to add some whitener (I like real cream, but that's another story …)? Before doing that,"take a moment to click a metal spoon against the side of your mug. You'll notice the sound is almost a musical note with a definite pitch. But as you add [ … ] the whitener and stir [ … ] the pitch of the clink changes. It can drop by an octave or more. Until 1969 no theories had been put forward to account for this phenomenon [ … ] Finally a group of scientists [ … ] suggested that air bubbles were the key [ … ] Particles of [ … ] whitener have, trapped on their surfaces, tiny bubbles of air. These are released when the powder dissolves in the water. Sound travels more than four times slower in air than water [ … ] Even if the bubbles make up only one one-hundredth of the total volume of the coffee [ … ] the speed of sound drops thirty-fold as compared with plain water. [ … ] The pitch of a note is actually a measure of the number of sound waves per second. So when the the speed of sound drops in the coffee mug, there are fewer vibrations per second, and the pitch of the mug's clink drops."
[ from Jay Ingram, The Science of Everyday Life, p. 28 - 29 ]
Readings, Resources and Questions
-
The title of this lecture is taken from Pellegrino Artusi, Science in the Kitchen and the Art of Eating Well,
U of Toronto Press, Toronto, 2003. This is the English translation, by M Baca and S Sartarelli, of a famous cookbook ("the
first written in Italian for the home cook"), La Scienza in Cucina e l'Arte di Mangiare Bene, published in 1891.

Pellegrino Artusi, Science in the Kitchen and the Art of Eating Well
-
For further information on mayonnaise, read also
 What is Mayonnaise?
What is Mayonnaise?
- Coffee has a very long history. As you can read in Coffee , "Coffea Arabica was discovered growing wild on the plateaus of central Ethiopia around 600 AD. The tree was found in Yemen on the southern tip of the Arabian Peninsula." See also The History of Coffee. This page is part of the interesting Mr. Cappuccino website.
-
Another great book on the science of cooking is of course Harold McGee's On Food and Cooking: The Science and Lore of the Kitchen, Scribner, NY 1984, 2004,
which was mentioned at the beginning of this lecture.
Here is a brief list of additional resources:
- Jay Ingram, The Velocity of Honey and More Science of Everyday Life, Viking Canada, 2003
- Sidney Perkowitz, Universal Foam: From Cappuccino to the Cosmos, Walker & Co., NY 2000
- A J Haagen-Smit, Smell and Taste, in Scientific American, March 1952. Reprinted in Scientific American Reader, Simon & Schuster, NY 1965
- Food and Science
- Food, With a Side of Science
- Chemistry's Everywhere! Show
- Food Chemistry
- How Bread Works
- An Introduction to Molecular Mixology
- Kitchen Chemistry
- Science of Cooking
© Copyright Luigi M Bianchi 2003-2005
Picture Credits: Faceblind.org · UIUC · Stanford U
U of Maryland · Cornell · Forlimpopoli
Last Modification Date: 27 April 2007
Picture Credits: Faceblind.org · UIUC · Stanford U
U of Maryland · Cornell · Forlimpopoli
Last Modification Date: 27 April 2007